A, the type of gas production
Flexible packaging lithium-ion battery gas generation is divided into two types of normal gas production and abnormal gas production. Normal gas production refers to the formation process of SEI film during the production process of the cell, often referred to as formation gas production. This gas can generally be stored temporarily in an air bag and discharged in the subsequent process, with no significant impact on the core.
Abnormal gas production means that when the gas bag is removed and the encapsulation is completed, there is too much gas due to an abnormality inside the battery, in which case the gas cannot be discharged, causing the cell to bulge, affecting the use of the appliance and causing deterioration in the performance of the cell. When the internal pressure is too high, it will easily open the packaging foil, causing serious damage such as leakage and corrosion. Therefore, understanding the entire gas production process of the cell and preventing abnormal gas production is the key to the production of flexible packaging lithium-ion batteries.
1 Formation gas production
Formation gas production refers to the formation process in the cell manufacturing process, that is, the first charging process of the battery, the electrolyte in the electrode surface oxidation, reduction reaction, the formation of solid electrolyte film (SEI film) accompanied by gas production; China Electronic Science and Technology Group Corporation, the eighteenth research institute of Chen Yikui, etc. study the positive electrode, negative electrode gas production comparison and gas composition analysis, it is concluded that the battery out of the formation stage gas production is mainly The gas production is mainly concentrated in the negative electrode of the battery. Huang Li et al. from the Baolong Institute of Battery Research, Xiamen University, studied in detail the types and quantities of gases produced at different formation voltages.
The results showed that below 2.5V, the gas produced was mainly H2 and CO2; after 2.5V, a small amount of EC started to decompose and the products were mainly C2H4; after 3V, DMC and EMC in the electrolyte started to decompose and the gas produced contained alkanes such as CH4 and C2H6 in addition to C2H4; above 3.8V, the product of EC decomposition, C2H4, basically disappeared. The voltage is between 3.0 and 3.5 V, and the gas production of the chemistry process is the largest, indicating that at 3.5 V, it is the main film-forming region of the SEI membrane.
SEI membrane ion-conducting electron non-conducting, in the structure consists of two layers, the inner layer is a dense and stable inorganic layer, the outer layer is a porous and loose organic layer, the thickness between 2nm to tens of nanometers, the outer organic product layer, with a certain flexibility, can improve the mechanical strength and integrity of the entire membrane layer, effectively blocking the solvent molecules in the electrode surface continued reduction reaction, therefore, after 3.5V due to The gas production is basically completed after 3.5V due to the blocking effect of the SEI film and the gas production decreases rapidly. The decomposition of EC during the formation of the SEI membrane consists of two types of one-electron reactions and two-electron reactions:
Where the one-electron reaction forms lithium alkyl carbonate and is accompanied by the production of large amounts of ethylene gas as shown in (1) and (2) below;
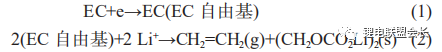
The two-electron reactions form mainly lithium carbonate and CO gas, as shown in reactions (3) to (5):
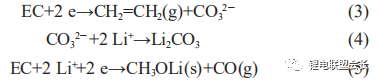
Better electrolyte and suitable material matching can produce excellent and stable SEI film, which not only can effectively block the decomposition of electrolyte and improve the first time efficiency, but also the subsequent amount of gas generated due to the dissolution and regeneration of SEI is less, so the effective selection of material and electrolyte system can reduce the amount of chemical gas production and improve the comprehensive performance of the battery.
2 Abnormal gas production
In the production process of soft pack batteries, there are many factors that can lead to abnormal gas production, which are divided into several categories: firstly, the film formation of the cell itself is unstable, and during the subsequent cycle, the SEI film on the surface of the negative electrode may fall off or become loose and undergo SEI film reconstruction, accompanied by gas production; secondly, the water content inside the cell is excessive; thirdly, the short circuit inside the cell leads to abnormal gas production; fourthly, the high temperature storage process gas production; five is overcharge and overdischarge gas production.
The dense and stable film is the prerequisite for the performance of the battery cell, while the excessive water content and the short circuit inside the battery are frequent problems in the production process. The following will be a brief analysis of these situations.
Second, abnormal gas production cause analysis
1 Water content abnormal flatulence
Due to the sensitivity of the entire battery system to moisture, although there is a lot of research that the presence of traces of water generated by the LiF makes the SEI membrane performance more stable, but when there is an excess of water, not only the consumption of lithium salt increases, reducing battery performance, but also accompanied by a large number of gas generation, so that the battery gas bloating, resulting in battery failure, when the negative electrode has lithium precipitation encounter water will occur violent reaction to generate heat triggered When lithium is precipitated from the negative electrode, it will react violently with water and cause more serious safety problems. Therefore, moisture control is a prerequisite for the production of lithium-ion batteries, and is also a process parameter that needs to be strictly controlled during the production of flexible packaging batteries.
The performance of moisture-exceeding cells is focused on two points: firstly, the hydrogen content of the gas component is significantly increased, and secondly, from the perspective of the chemical capacity, the capacity of flatulent cells is smaller than normal cells. This is due to a series of reactions that occur inside the core, resulting in a large amount of side reaction gas generation and causing flatulence. Firstly, the water itself is electrolysed during charging, producing hydrogen gas, as shown in reaction (15), and secondly, the water reacts with the lithium salt in the electrolyte to produce hydrogen fluoride gas, which also corrodes the aluminium foil.
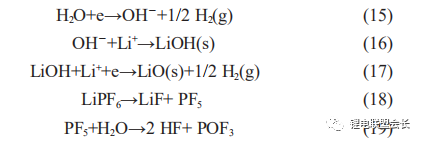
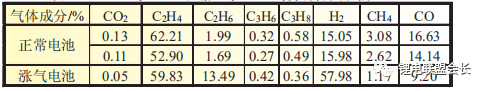
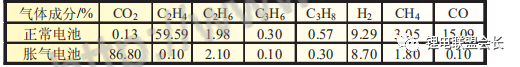
It is clear from the comparison of the gas composition analysis in Table 1 that the hydrogen content in the bloated core due to the abnormal water content is significantly higher and that the HF generated during placement is highly susceptible to corrosion reactions with the aluminium foil, therefore the presence of HF in the gas composition is not detected.
The introduction of the water content allows for side reactions to occur within the cell, causing interfacial damage and increased polarisation during the chemistry process, making it easy to reach the charging potential, making the charging time generally smaller than normal cells and therefore the charging capacity smaller than normal (Figure 1).
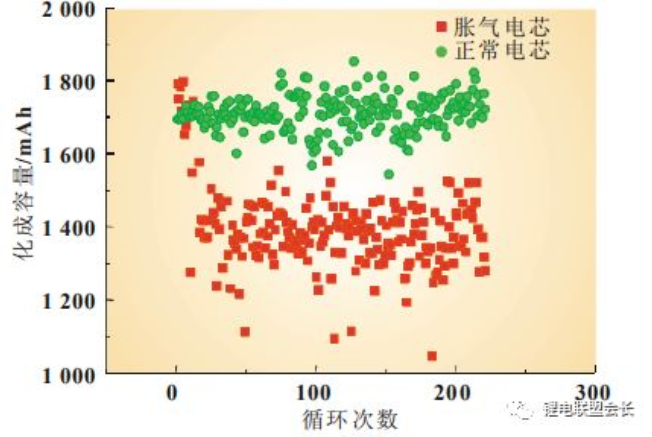
Figure 1 Comparison of the charging capacity of flattened and normal cells
The water content inside the cores exceeds the standard, triggered by a variety of reasons, but can be roughly divided into two main categories: one is due to poor encapsulation, the subsequent moisture in the air into the cores inside the lead; the other is caused by poor control of moisture during the production process, such as the diaphragm is not dried before the electrolyte injection operation; drying room water content exceeds the standard; electrolyte in the use process introduced moisture, etc. As shown in Figure 2, with the increase of time, the water content inside the bare core increases. The bare core after vacuum baking should be sealed with liquid injection in time to prevent the water from re-entering the diaphragm with the growth of placing time, leading to the subsequent swelling.
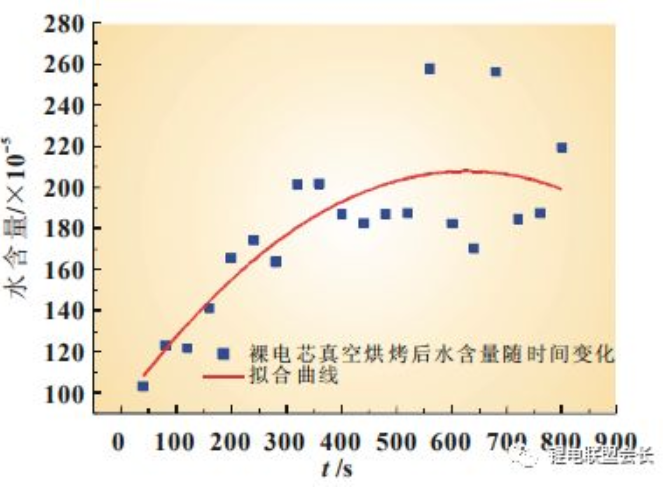
Fig. 2 Change curve of water content of bare core after vacuum baking with time increase
2 Internal short circuit flatulence
During the production process, when an internal short circuit occurs, the local temperature rises sharply, leading to the decomposition of the electrolyte. Analysis of the gas composition of such a distended cell reveals a significant increase in CO2 content, which is due to the reaction between the electrolyte and PF5, the decomposition product of LiPF6, in the presence of high temperature and trace water (20) and (21), resulting in a significant increase in the amount of CO2 and the occurrence of gas distention.
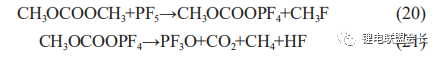
As the temperature can reach over 200°C when an internal short circuit occurs and causes the diaphragm to burn and carbonise, such cells can generally be disassembled to find burned short circuit points, and the oxygen generated by the decomposition of positive Li0.5CoO2 at high temperatures accelerates the decomposition of EC, the main solvent in the electrolyte, as shown in the reactions below, making gas bulging generally more severe (Table 2).
Table 2 Comparison of the gas composition of normal and bloated cores
3 High temperature storage and overcharge and overdischarge bloating
During high temperature storage and overcharge and overdischarge, LiCoO2 is in a sub-stable state and is extremely unstable, resulting in the following decomposition reactions, as shown in (22) to (24):
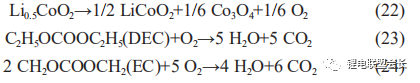
In addition, when the stability of the SEI film is low, the organic layer of the outer layer of the film in contact with the electrolyte will dissolve as the temperature rises. For example, (CH2OCO2Li)2, as the main component of the lithium alkyl ester layer of the SEI film, is very unstable and prone to decomposition reactions as in (25), generating gas and bulging of the cell;
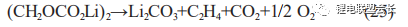
III. Measures to suppress abnormal gas production
In the normal voltage range, the amount of gas production is small and mostly hydrocarbons, when there is abnormal gas production occurs, a large amount of gas will be generated, destroying the electrode interface structure, leading to electrolyte decomposition failure, and in serious cases break through the encapsulation area causing leakage and dangerous corrosion. The suppression of abnormal gas production requires a combination of material design and manufacturing processes.
The first step is to design and optimise the material and electrolyte system
To ensure the formation of a dense and stable SEI film, the stability of the cathode material is improved and abnormal gas production is inhibited. The electrolyte is often treated with a small amount of film-forming additives to make the SEI film more homogeneous and dense, reducing the risk of the SEI film falling off during use and of the battery bulging due to gas production during regeneration.
Related research has been reported and applied in practice, for example, Cheng Chang et al. from Harbin University of Technology reported that the use of film-forming additives VC can reduce the phenomenon of battery gas swelling. However, most of the research has focused on single component additives with limited effect. Cao Changhe et al. from East China University of Science and Technology, used VC and PS compound as a new electrolyte film-forming additive and achieved good results, with significant reduction in gas production during high temperature shelving and cycling of the battery.
Studies have shown that the SEI film component formed by EC and VC is linear lithium alkyl carbonate, and the alkyl lithium carbonate attached to LiC is unstable at high temperatures, decomposing to produce gases (such as CO2, etc.) and producing cell bulging. The SEI film formed by PS is lithium alkyl sulfonate, which has a defective film, but has a certain two-dimensional structure and is still stable under high temperature when attached to LiC. When VC and PS are used together, at low voltage PS forms a defective two-dimensional structure on the negative surface, and as the voltage rises VC forms a linear structure of lithium alkyl carbonate on the negative surface, which fills in the defects of the two-dimensional structure and forms a stable SEI film with a network structure attached to LiC. This structure of the SEI film greatly improves its stability and can effectively inhibit gas production due to membrane decomposition.
In addition, the interaction between the cathode lithium cobaltate material and the electrolyte makes its decomposition products catalyse the decomposition of the solvent in the electrolyte, so the surface coating of the cathode material can not only increase the structural stability of the material, but also reduce the contact between the cathode and the electrolyte and reduce the gas generated by the catalytic decomposition of the active cathode. Therefore, the formation of a stable and complete cladding layer on the surface of the cathode material particles is also a major development direction at present.
The next step is to strictly control the manufacturing process parameters
To ensure the reliability of the package and to prevent swelling caused by excessive moisture inside the battery, the control methods are as follows:
(1) Adequate drying of the cells after winding is completed to prevent excessive moisture content in the diaphragm;
(2) Strictly control the time from cell to liquid injection after vacuum baking and the humidity in the drying room;
(3) Ensure the sealing of the liquid-filled glove box;
(4) Controlling the moisture and free acid content in the electrolyte;
(5) Regulating the electrolyte storage environment and sealing conditions to prevent excessive moisture from entering the electrolyte during use and storage;
(6) Use of closed-port pressurisation or external airbag formation followed by vacuum sealing and venting;
(7) Multi-step formation and high temperature shelving processes to ensure complete gas production;
(8) Improved packaging reliability.


 Home
Home CSIP
CSIP  Apr 12,2023
Apr 12,2023 
 Technology丨The assembly process of soft pack lithium battery is illustrated-CSIP
Technology丨The assembly process of soft pack lithium battery is illustrated-CSIP 
 Mar 01,2023
Mar 01,2023 













